Monitoring Relative Humidity in Controlled Environments
Relative Humidity and Controlled Environments
Relative Humidity (RH) is one of many important variables monitored and controlled in cleanrooms, negative and positive pressure rooms and generic controlled environments. Some specific types of controlled environments, such as USP 797 pharmaceutical-based rooms require close monitoring and control of relative humidity; other industries monitor and control humidity as a means of preserving product efficacy and quality.
Basically, relative humidity (RH) is the amount of moisture in the air compared to what the air can “hold” at the current temperature…
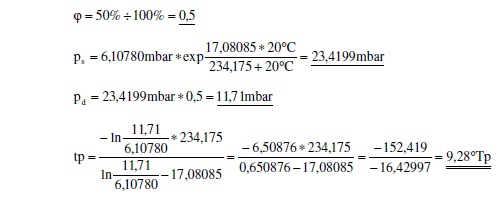
More precisely, relative humidity is the actual vapor density divided by the saturation vapor density multiplied by 100%. So, an example would be:
Readings from measuring instruments: temperature = 20.0 °C
Relative Humidity = 50%
In semiconductor cleanroom operations, relative humidity is held to much stricter standards. Typical values range from 30 to 50 percent with tolerances as narrow as ± 1 percent for some areas, such as photo-lithography. Ranges may be even less for deep ultraviolet processing (DUV).
An often overlooked capital expense within cleanroom operations is maintaining desired relative humidity ranges in makeup air. All too often, expenses associated with maintaining adequate filtration, and keeping low particulate counts are the focus of controlled environment design and operation, while variables such as temperature and relative humidity are considered. This can be a major miscalculation. If humidity gets out of control product integrity can also get out of control.
Why it matters
So, why go through all of the planning and expense to build and maintain a controlled environment and devote so much time to monitoring relative humidity? Quite simply because relative humidity affects not only room temperature (another important variable) but could degrade overall cleanroom performance and even potentially adversely affect the health and well being of cleanroom personnel and the general public.
Failure to maintain and control relative humidity could result in:
- Metal corrosion
- Static charge buildup
- Moisture condensation
- Photolithographic degradation
- Water absorption
- Bacteria growth
- Mold growth
- Pathogen growth
- Personnel comfort
- Temperature control
- Filtration effectiveness
- Fungi growth
- Prevent/Cause chemical reactions
- Affect adhesives and solvents
- Dirtier overall controlled environment
- Static electricity
Preventing Adverse Effects
In environments where relative humidity is at or above 60% it can be especially difficult to control or prevent the growth and contamination of:
- Bacteria
- Mold
- Fungii
- Viruses
Conversely, relative humidity lower than 30% can cause personnel discomfort such as:
- Dry and cracked skin
- Dryness in the eyes and mucous membranes
- Respiratory discomfort
- Electrostatic buildup and discharge
A compromise for both personnel comfort and product safety and efficacy is in the 40% to 55% range. In cases where more or less humidity is required that may result in personnel discomfort, additional safety procedures are taken to prevent electrostatic buildup and discharge. An example would be in semiconductor manufacturing.
Another consideration is in cleanroom operations where chemical reaction are taking place. Increases and decreases in relative humidity can result in undesired curing processes for epoxies and other chemical compositions – especially in the aviation industry or other manufacturing processes where adhesion is important to end-user safety.
Keeping Cleanrooms Clean
In controlled environments, and especially cleanrooms, cleanliness is the primary focus. Keeping particulate counts low is the name of the game. While expensive filtration devices and precisely engineered HVAC systems are important, noting on earth can completely eliminate all particulates from a given space. That’s right, with current physics, it is impossible. So, some particles will escape the filtration system and end up in the room; however, once in the room, relative humidity can affect the behavior of these particles in a process known as the Kelvin Condensation Effect (capillary condensation and adhesion).
When the relative humidity is higher, in the range of 60-70% or more, capillary forces creates a bonding bridge between a surface and containment; increasing particle adhesion to substances such as silicone. In some controlled environments, which require higher humidity to operate, micro-dimensional surfaces help to counteract this naturally occurring adhesive process.
Higher humidity can also create longer particulate airborne time. Denser air means particles of certain size can stay airborne longer, and thereby affect controlled room processes for longer periods of time.
Long-term Considerations
When designing and operating cleanrooms and controlled environments, one thing is for certain, no matter what you do, there will always exist the potential for unwanted dirt and particles which increases the risk to, not only personnel and the general public, but also to the processed going on in the room. The key is reducing this risk. In this case, it is very true “what you don’t know can hurt you.” Being aware of what environmental conditions, temperature and relative humidity, exist in your controlled environment at all times is critical.
There are dozens of environmental monitors on the market which measure relative humidity. Some of these instruments also monitor other variables such as temperature and differential pressure. The most important consideration is precision. In a critical/controlled environment, never purchase a product based on cost. Your instrumentation should be based on a proven track record of success, and already used by companies that have a lot to lose. Look at the instrument spec sheets, compare with similar products on the market. If possible, call the company and see if you get a live person. If the company does not have real people answering the phone during the sales process it is a pretty good bet that you will not get a real person on the phone when you need support. You may also want to ensure your instrumentation is easy to set up, install, and monitor.
Ease of Use
Chances are, if your instrument is too difficult to use and/or understand, it won’t be used at all. Think of a relative humidity monitor as a safeguard between your company, products, personnel and end users. Explore your options. If you already have a relative humidity monitor in place, make sure you have the best you can possibly afford; one that gives advanced warning in the case of potentially unwanted room conditions. Millions and millions of dollars are lost each year because of compromised controlled environments. This is completely avoidable if your cleanroom or controlled environment can provide advanced warnings to operators and personnel.