Negative Pressure Rooms
Negative Pressure Rooms: Protecting People
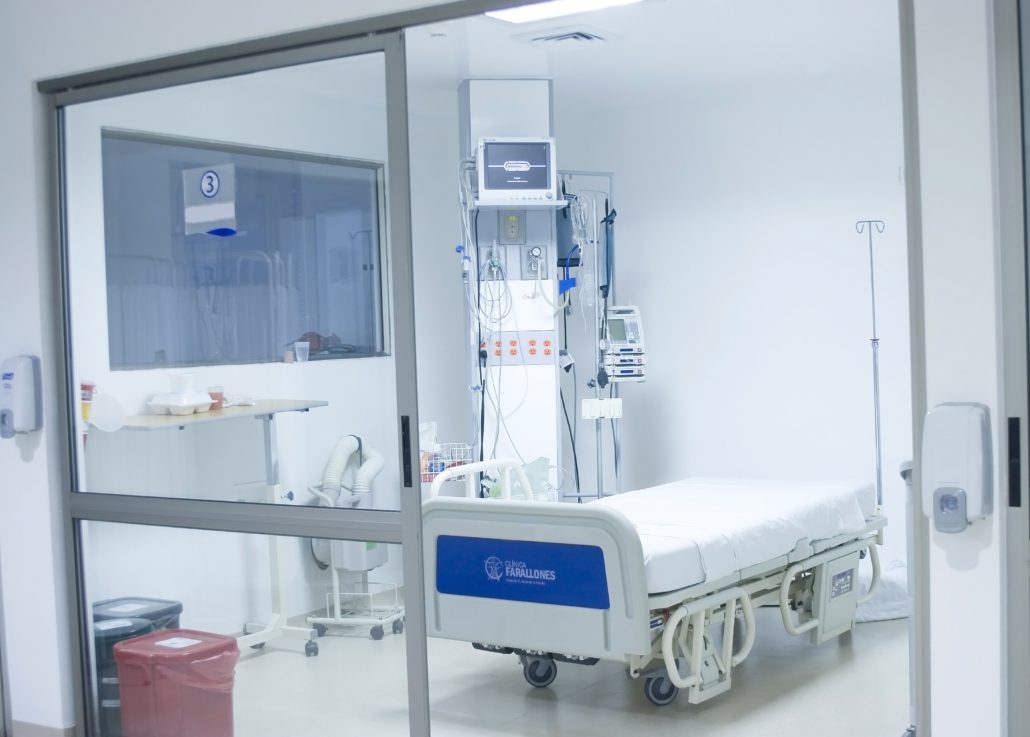
Negative Pressure Rooms are used primarily in hospitals and bio-tech facilities to ensure staff and patients are safe from cross contamination from airborne disease and other potentially dangerous contaminants. Negative Pressure Rooms are very different from standard cleanrooms whereas standard cleanrooms are designed to push air out when an air lock is opened, or have “positive pressure” at all times.
In a hospital setting, certain populations are more vulnerable to airborne infections including immune-compromised patients, newborns and elderly people. Of course, hospital personnel and visitors can also be exposed to airborne infections as well.
A negative pressure room in a hospital is used to contain airborne contaminants within the room. Harmful airborne pathogens include bacteria, viruses, fungi, yeasts, molds, pollens, gases, VOC’s (volatile organic compounds), small particles and chemicals. This is just part of a much larger list of airborne pathogens present in a hospital or laboratory environment.
Rooms that should be negatively pressurized according to The 2014 FGI Guidelines/Standard 170-2013 include:
- ER waiting rooms
- Radiology waiting rooms
- Triage
- Restrooms
- Airborne infection isolation (AII) rooms
- Darkrooms
- Cytology, glass washing, histology, microbiology, nuclear medicine, pathology, and sterilizing laboratories
- Autopsy rooms
- Soiled workrooms or holding rooms
- Soiled or decontamination room for central medical and surgical supply
- Soiled linen and trash chute rooms
- Janitors’ closets
A negative pressure isolation room is commonly used for patients with airborne infections. For example, a patient with active/live tuberculosis, a disease caused by the bacteria Mycobacterium tuberculosis, will be placed in a negatively pressurized room because the tuberculosis bacterium is spread in the air from one person to another.
Using a negative pressure room can better contain the bacterium within the room.
Negative Pressure Room Basics
In a Negative Pressure Room, once an airlock is opened, air is only permitted to enter the room so any contaminants in the room can not escape. Constant vacuum pressurization inside a Negative Pressure Room allows it to maintain the suction at a specific pressure rating; thereby protecting anyone outside of the room from contaminants that could escape from inside the room.
Negative Pressure Rooms are nothing new. In fact a typical bathroom with a closed door and exhaust fan running is a type of Negative Pressure Room. In hospitals, however, the setup is much more complex and much, much cleaner. In hospitals, negative pressure is maintained by balancing the room’s ventilation system so more air is mechanically exhausted from a room than is supplied by the exterior/surrounding building HVAC system. When constructed correctly, this creates a ventilation imbalance, which the room ventilation compensates for by continually drawing air in from outside the room.
In a well-designed negative pressure room, this air is pulled in under the door through a gap. This gap is typically about half an inch high. Other than this single gap, the room is almost always air tight to prevent air from being pulled in through undesired cracks and gaps, i.e. around windows, light fixtures and electrical outlets. Leakage from these areas can compromise (or eliminate) room negative pressure, even if the system is balanced to achieve it. Think of these as small holes in the hull of a boat; eventually it will matter, even if the largest of them is plugged.
Overall, the minimum pressure difference necessary to achieve and maintain negative pressure that will result in air flow into the room is actually very small (0.001 inch of water gauge/ “w.c.). The actual level of negative pressure differential will depend on the difference in the ventilation exhaust and supply flows and the physical configuration of the room.
If a room is well sealed, negative pressures greater than the minimum of 0.001 inch of water are easily accomplished. However, if rooms are not well sealed, as typical with many facilities (especially older buildings with retro-fitted negative pressure rooms), achieving higher negative pressures may require exhaust/supply flow differentials beyond the standard ventilation system’s capacity. In these instances, contractors may have a custom Heating, Ventilation, Air Conditioning (HVAC)/air handler system designed to accomplish the desired result using multiple standard air handlers and filtration systems.
To establish negative pressure in a room that has a normally functioning ventilation system, the room supply and exhaust air flows are first balanced to achieve an exhaust flow of either 10% or 50 cubic feet per minute (cfm) greater than the supply (whichever is greatest). In 90% of cases, this specification should achieve a negative pressure of at least 0.001 inch of water. If the minimum 0.001 inch of water is not achieved and cannot be achieved by increasing the flow differential (within the limits of the ventilation system), the room most likely has some form of leakage (e.g. through doors, around windows, plumbing and equipment wall penetrations), and action should be taken to inspect and seal the leaks. Finding these leaks is accomplished using a simple smoke test (see below) in the room.
Negative pressure in a room can be altered by changing the ventilation system operation or by the opening and closing of the room’s doors, corridor doors or windows. Some rooms are outfitted with special plenums that can be electronically opened and closed based on digitally-acquired feedback systems that monitor the rooms environmental conditions.
What is a smoke test?
A smoke test is a simple procedure to determine whether a room is under negative pressure. The smoke tube is held near the bottom of the negative pressure room door and approximately 2 inches in front of the door. The tester generates a small amount of smoke by gently squeezing the bulb. The smoke tube is held parallel to the door, and the smoke is exhausted from the tube slowly to ensure the velocity of the smoke from the tube does not overpower the air velocity. If the room is under negative pressure, the smoke will travel under the door and into the room. If the room is not under negative pressure, the smoke will be blown outward or remain stationary.
Monitoring Negative Pressure Rooms
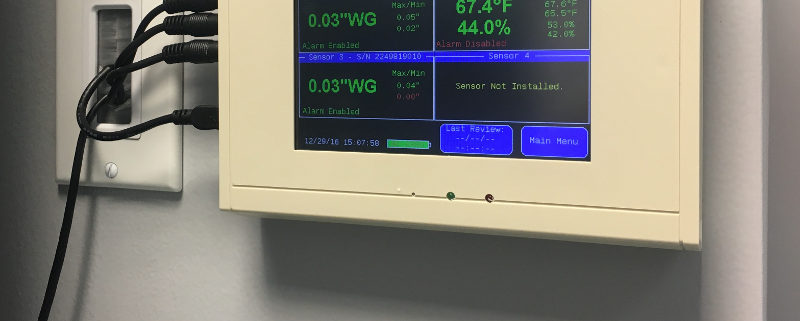
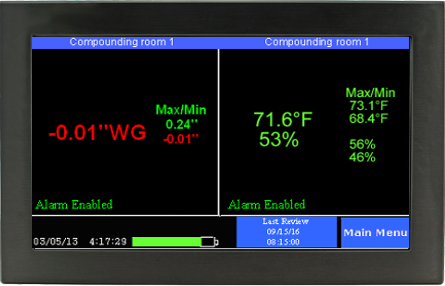
As important as maintaining negative rooms pressure, is continuously monitoring the systems which enable it. Without constant monitoring and validation, there is no immediate or physical way to verify proper room negative pressure levels. Even the slightest variations in air flow and variables that may affect it can severely disrupt proper pressurization, and potentially endanger staff, room personnel, and other innocent populations. A complete solution for monitoring negative room pressure would include functionality that would allow operators to quickly glance at a color display that reported both current room variables as well as historic room variables. These are sometimes referred to as “data loggers.” Additional features would include immediate reporting of room conditions such as temperature, relative humidity (RH) and room pressure.
If levels fall below or rise below specified values, becoming ‘unsafe’, a room alarm would notify personnel. Additional warnings would include a way to receive SMS/text alerts, email warnings and automated phone alerts to parties involved directly with the compliance and daily operation of the negative pressure room.